Abstract
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy of childhood, with a current incidence across all age groups of approximately 5,000 cases annually in the US and about 7,000 in Europe. Approximately 60% of cases are diagnosed in patients <20 years of age and approximately 85% of pediatric ALL derives from B-cell lineage. In the last decade, the introduction of cellular therapies in pediatric B-lineage ALL has changed the landscape of management, particularly for relapsed/refractory disease. Chimeric antigen receptor T-cell (CAR-T) based approaches allow the engineering of autologous patient T cells to specifically target and destroy tumor cells in a major histocompatibility complex (MHC)-independent manner. With the introduction of these novel therapies for ALL, cost of care is a critical component and financial toxicity experienced by patients and society requires evaluation.
Methods
Using de-identified commercial insurance data from the Optum Labs Data Warehouse, a cohort of 37 patients, aged 1-25 years, with ALL who received CAR-T therapy was identified, with a CAR-T product administration code between Oct 2016 and Dec 2021 in the United States. ALL diagnosis was confirmed based on ICD-9 and ICD-10 diagnostic codes, in combination with CPT codes for CAR-T administration. Mean and median cumulative costs were computed for a 90 day period, including 30 days prior to product administration, to avoid the variation of bridging therapies, and 60 days following CAR-T administration. Cost was further evaluated by the type and location of service provided, the presence of complication codes for cytokine release syndrome (CRS) and/or immune effector cell-associated neurotoxicity syndrome (ICANS). Results were stratified by age group (1-9 years vs. 10-25 years) as a proxy for upfront ALL risk group. Total cost was also evaluated based on outpatient vs. inpatient CAR-T administration.
Results
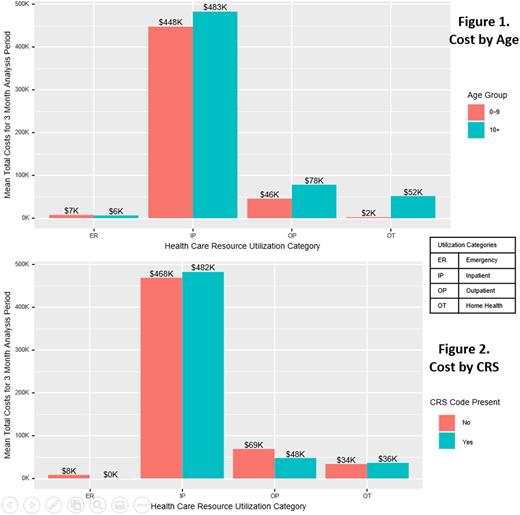
Among the 37 identified ALL patients that received a CAR-T product infusion (1-9 years, 13; 10-25 years, 24), 14 patients were female, 37.8% were White, median age at administration was 13 years (interquartile range 7-19 years) and 35 of 37 enrolled patients had continuous coverage enrollment from one month prior to infusion through two months post-infusion. Mean 90-day total cost of care was $585,398.10 (Median: $620,500.40). Inpatient cost accounted for approximately 71% of the total cost. Although inpatient cost was slightly higher in the older age group and in patients with a code for CRS, these differences were not statistically significant (Figure 1 & 2). No patients in the dataset had a diagnostic code for ICANS. Six patients died during the 60 days post-infusion.
Conclusions
This robust real-world cost analysis shows for the first time the true cost of CAR-T therapy for pediatric ALL - encompassing not only the cost of the cellular therapy product itself, but the care involved in providing it. We showed that the cost of care is not significantly impacted by patient age or the incidence of CRS. Mean total cost well exceeded $500,000 US dollars, as expected with the listed commercial price of tisagenlecleucel of $475,000. This study provides a valuable benchmark that can be used to analyze the financial toxicity of CAR-T therapy for pediatric ALL therapy on health systems, patients and families. The cost of this therapy can be re-assessed over time as other novel therapeutics are introduced into ALL therapy and long-term outcome data for this therapy are established.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal